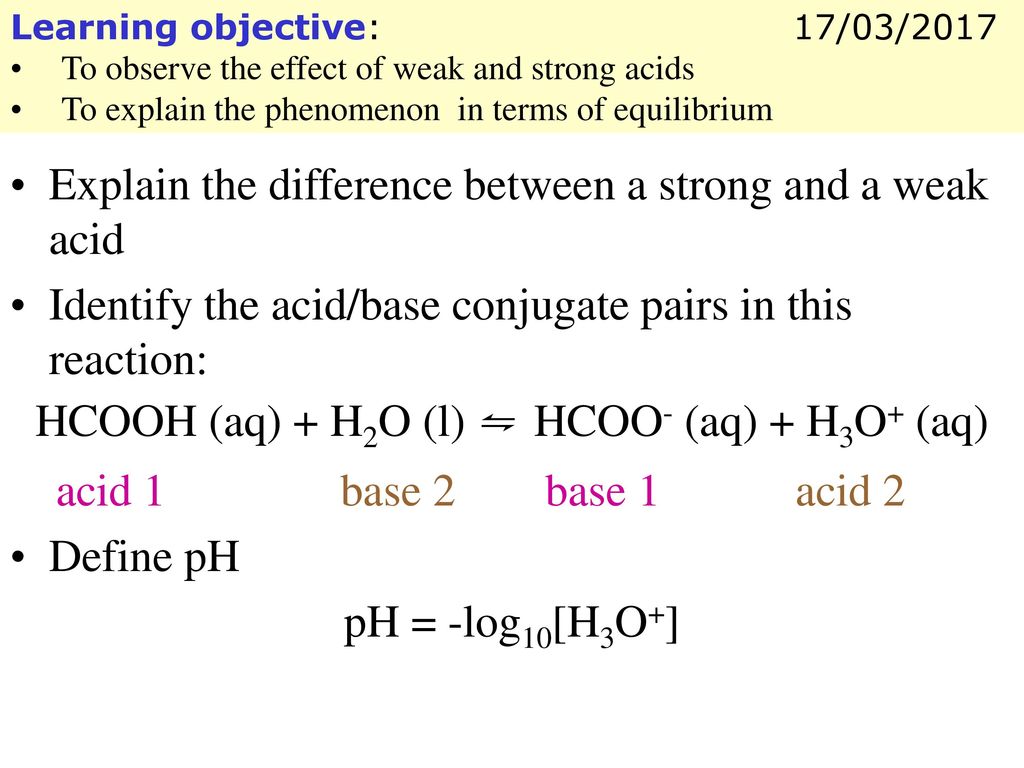
Explain Difference Between a Strong Acid and a Weak Acid
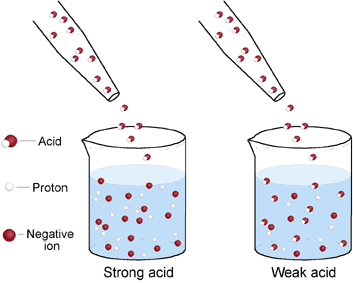
Strong acids are molecules that completely dissociate into their ions when it is in water. A strong acid and a weak acid of the same concentration.

Strong Weak Acids And Bases Youtube
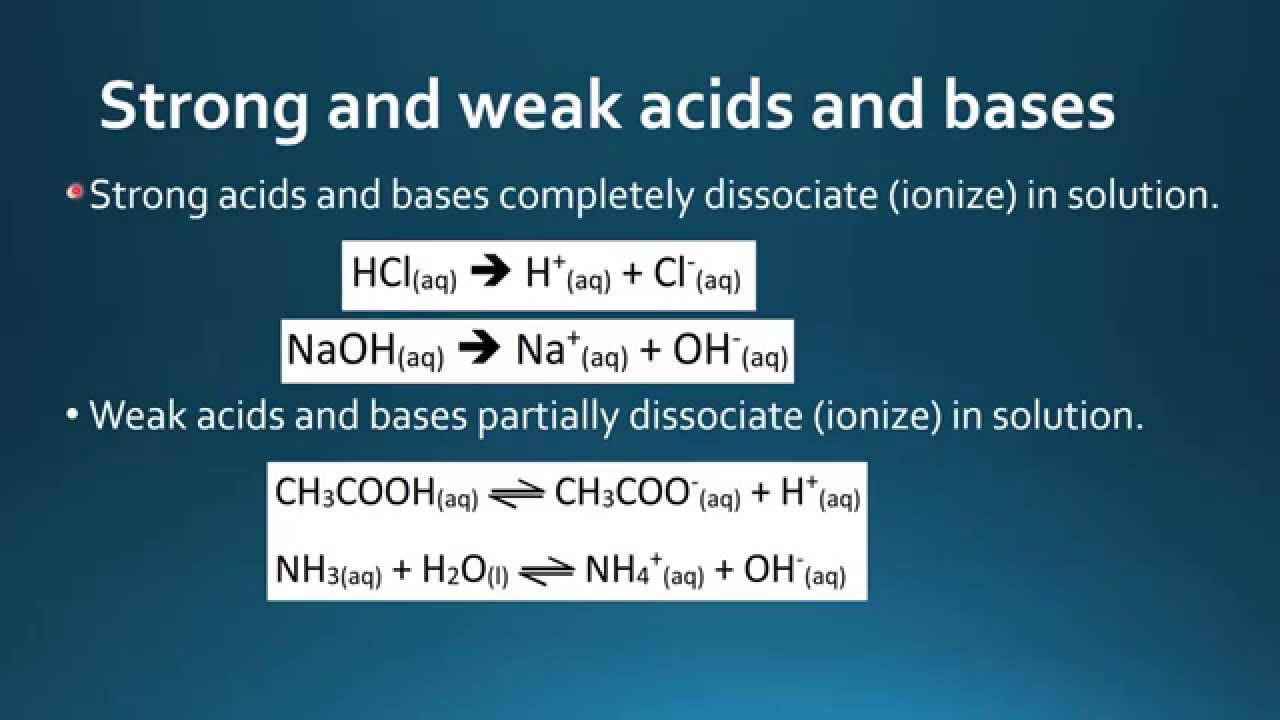
HCl aq H aq Cl-aq All of the HCl molecules becomes into hydrogen ions chloride ions when they are dissolved in water.
. Strong acids and bases produce a higher number of ions in a water solution than weak acids and bases. Will react at different rates with the same metal. Submitted by katielady on Tue 06052012 - 1655.
An example is HF. A strong acid is an acid that completely ionizes in solution whereas a weak acid is an acid that does not completely ionize in water. And this amount of association is determined by the K which is a very important um value for acids.
Strong acids are good conductors of electricity on contrary weak acids are not too good as conductors of electricity. Strong acids have a higher concentration of H ions and fully ionize. Complete the table given bellow.
Explain the difference between a strong acid and a weak acid. A weak acid is only partly less than 100 ionized. But we Cass is they partially associates.
So strong assets completely. You can see the difference between a strong acid and a weak acid. What is the difference between strong and weak acids.
Explain and use A examples of each type of acid. An example is HCl. The strong acid reacts faster and you see more bubbles of hydrogen.
The pH of a weak acid. Up to 256 cash back Explain the difference between a strong acid and a weak acid using the two compounds below. Strong acid passes electricity faster whereas weak acids are slow conductors.
Strong acids are acids that completely dissociate in aqueous solutions releasing H ions. Of the same concentration by looking at the reaction with magnesium. Hydrochloric acid sulphuric acid.
A strong acid is one that does not completely ionize in water. A strong acid is completely 100 ionized. Difference Between Strong and Weak Acids Definition.
Acetic Acid Titration with NaOH. -HClO2aq a weak acid -HClO3 aq a strong acid. Strong and weak acids are not the same as concentrated and diluted acids.
Explain the difference between a strong acid and a weak acid. A weak acid only partially ionizes in solution. H3O OH- pH Acidic basic or neutral 10 x 103 on 30 x 10-6 neutral.
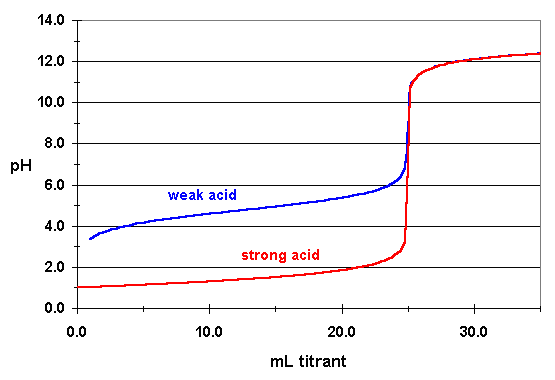
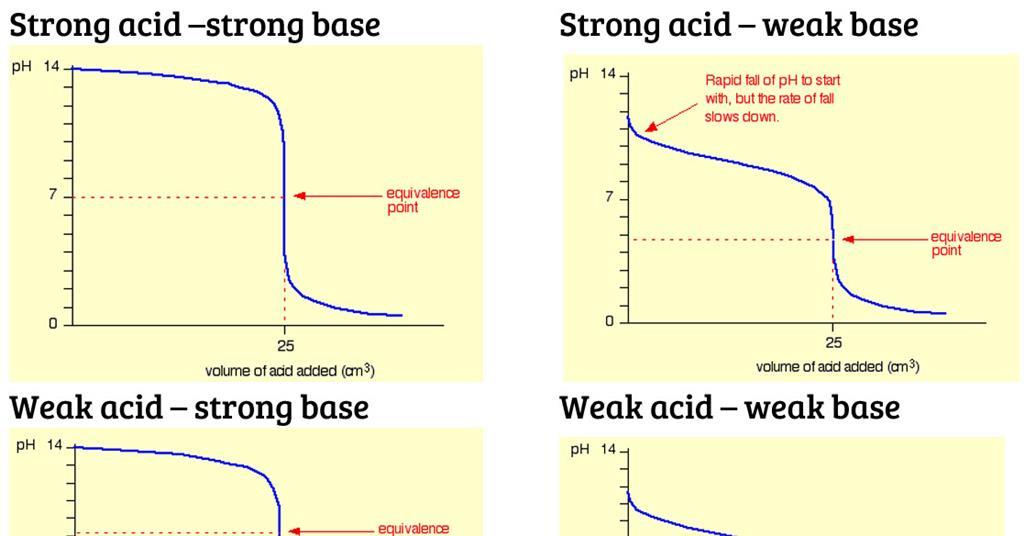
A Both the strong acid and the strong base are completely dissociated in solution whereas the weak acid dissociation equilibrium constant is small enough that there is a measurable amount of the un-dissociated acid present in aqueous solution. This means that the pH values of strong acids are lower than that of weak acids which explains why the rate of reaction of strong acids with substances such as. A strong acid is one that is 100 percent ionized in a solution and a weak acid is one that doesnt ionize fully when dissolved in water.
There are two major types of acids as strong acids and weak acids. Strong acid is an acid that ionizes completely in aqueous solution. Weak acids have a.
PH of weak acid is 3-5. The key difference between weak acid and dilute acid is that weak acid is a compound that partially dissociates when dissolved in water whereas dilute acid is a solution containing more water than acid. Write an equation for the disassociation of both hydrochloric and acetic acid in water.
Strong acids have a high dissociation constant while weak acid has a low dissociation value. I know the equivalance point for SA - SB pH is 7. It gives off only a few of its H atoms when dissolved in water.
For example H s Thats a weak acid and it only partially associates into HPE laws plus f minus. Main Differences Between Strong Acid and Weak Acid. Sulfuric acid is an example of a strong acid and hydrogen fluoride is a weak acid.
It always loses a proton H when dissolved in water. It completely dissociated into H plus and C l minus. B The equivalence point of a titration of a strong acid with a strong base may be observed with an indicator.
Will you put that into water for example H C l. Weak acid is an acid that ionizes partially in a solution. HCl Titration with NaOH.
A weak acid is one that completely ionizes in solution. The pH of a strong acid solution is very low about pH1. Weak acids are molecules that partially dissociate into ions in aqueous solution.
The main difference between strong acidsbases with weak acidsbases is that strong acid will have most of their molecules ionized. A strong acid has a pH 1. B- A Basic Buffer two components.
Key Difference Concentrated Acid vs Strong Acid An acid is a chemical compound that can release H ions to the medium where it resides through ionization of the acid molecule. Strong acid add all their H to will weak acid only add some H to solution. Weak acid comes under the classification of acids according to acid strength and the dilute acid comes under the category of concentration of acids.
Explain the reason for the difference between the equivalence points for strong acid and strong base and weak acid vs strong. A weak acid is an acid in which only a few molecules break apart when the acid is dissolved in water. Strong acids react faster whereas weak acids take time to react with any base.
Write chemical equations to support your answer. Strong acids and bases are better conductors of electricity than weak acids and bases. What is the difference between a strong acid and a weak acid.
Strong acid possesses higher conductivity due to presence of unpaired atoms. Strong acids completely disassociate in water whereas weak acids do not. And WA - SB pH is basic.
Strong acid is an acid that ionize completely while weak acid partially ionize. A strong acid completely ionizes in solution. A strong acid is an acid in which all ofthe molecules of the acid break apart when the acid is dissolved in water.
A- An Acid Buffer two components.

Acids And Bases I Introduction
Difference Between Pka And Ph Definition Values Relationship

Weak Acid Weak Base Reactions Video Khan Academy

8 3 3 Distinguish Between Strong And Weak Acids And Bases Youtube

Strong And Weak Acids And Bases Ppt Download
Acids Bases Strength Vs Concentration Pathwayz

Strong And Weak Acids Bases Video Khan Academy
Which Indicator Is Used In A Strong Acid Versus A Strong Base Solution Quora

Strong And Weak Acids And Bases Ppt Download

How To Determine If Acid Is Strong Or Weak Shortcut W Examples And Practice Problems Youtube
Difference Between Weak Base And Strong Base Difference Between
Difference Between Strong And Weak Acids Definition Properties Examples

What Is The Difference Between The Titration Of A Strong Acid With A Strong Base And That Of The Titration Of A Weak Acid With A Strong Base Chemistry Stack Exchange
Teaching Acids And Bases Post 16 Cpd Rsc Education
8 3 3 Distinguish Between Strong And Weak Acids And Bases Youtube
Comments
Post a Comment